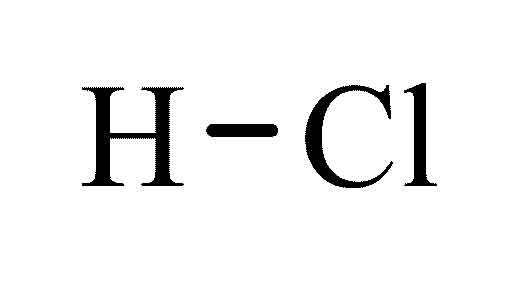
Hydrochloric Acid
Hydrochloric acid is a colourless liquid with a sharp, irritating odor. Consists of hydrogen chloride dissolved in water. It sinks and mixes with water, produces irritating vapor.
- Other Names
- Muriatic acid, spirits of salt, hydronium chloride, chlorhydric acid, chlorane, hydrogen chloride
- Chemical Formula
- HCL
- CAS No
- 7647-01-0
- Molar Mass
- 36.45 g/mol
| TEST | RESULT |
|---|---|
| Appearance | Clear, Colourless To Yellowish Liquid |
| Acidity (As HCl) | 30 - 33 % |
| Residue On Ignition | 0.01 % |
| Sulphate (As H2SO4) | 0.1 % - max |
| Iron (As Fe) | 0.02 % - max |
| Free Chlorine And Bromine | 0.002 % - max |
Applications:
Hydrochloric acid used in cleaning and pickling of metals.
Manufacture of chlorine and chlorides, manufacture of gelatine, food processing industries, etc.
In the production of high fructose corn syrup (HFCS), which is found in soft drinks, accounts for 70 5 of the demand.In leather industry for de-liming, tanning and drying of hides.
It is used to remove rust, scale and undesirable carbonate deposits in oil wells.
In pickling of carbon, alloy and stainless steels. It is also used in aluminium etching, metal prefixing for galvanising and soldering, and metal cleaning.
In the production of organic compounds, such as vinyl chloride and dichloroethane for PVC.In the regeneration of ion exchange resins.
In the removal of heavy metal from carbon black and activated carbon.In the manufacture of chlorine dioxide.
‘

