Nitric Acid
Nitric acid is a colourless, pale yellow or red fuming liquid generating rd fumes and having a suffocating odour. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects.
- Other Names
- Hydrogen nitrate, acidumnitricum
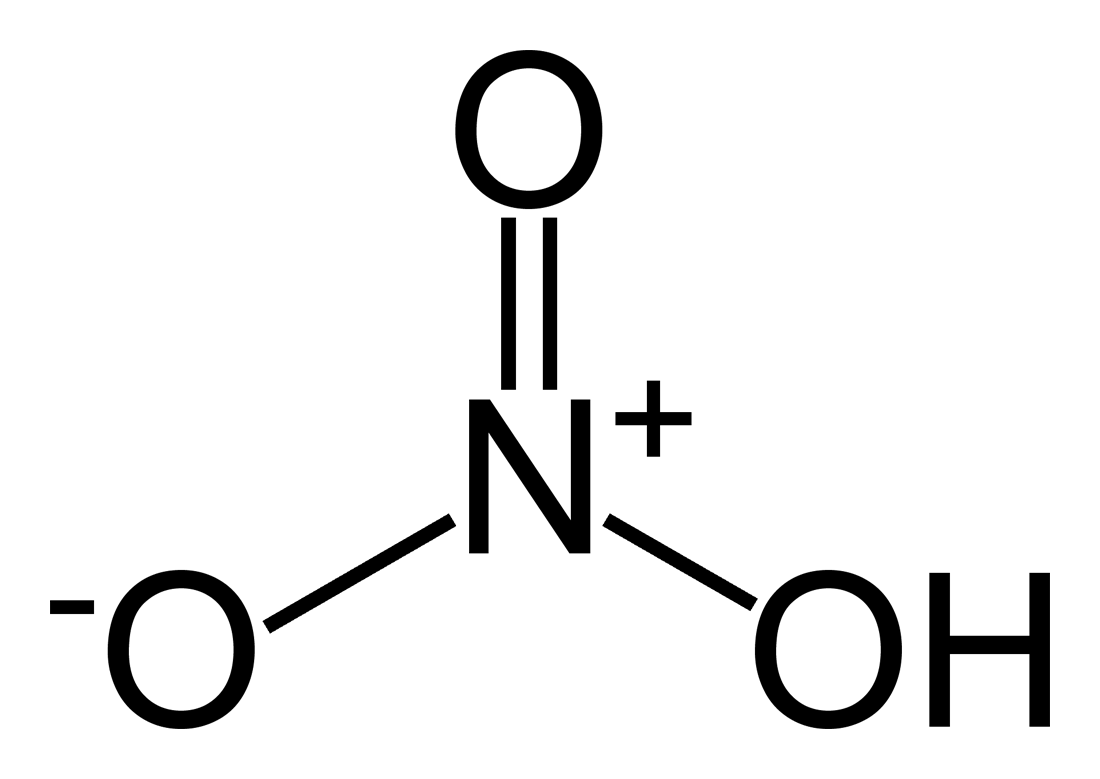
- Chemical Formula
- HNO3
- CAS No
- 7697-37-2
- Molar Mass
- 63 g/mol
| TEST | RESULT |
|---|---|
| Appearance | Clear, Colourless Liquid |
| Acidity (As HNO3) | 68 % |
| Specific Gravity (At 25 °C) | 1.4 |
| Nitrous Acid (As HNO2) | 0.01 % - max |
| Chlorides (As Cl) | 0.0002 % - max |
| Residue On Ignition | 0.002 % - max |
| Iron Content (As Fe) | 0.0001 % - max |
Applications:
It is used in various forms as the oxidizer in liquid-fueled rockets. These forms include red fuming nitric acid, white fuming nitric acid, mixtures with sulphuric acid, etc.
Nitric acid is used either in combination with hydrochloric acid or alone to clean glass cover slips and glass slides for high end microscopy applications. It is used in the manufacture of fertilizers and explosives.
It can be used to identify and review the purity of gold, particularly in low-grade alloys. It is highly used in the manufacturing of explosives.
Apidic acid is produced on a large scale by oxidation of cyclohexanone and cyclohexanol with nitric acid. It is used in the manufacture of organic synthesis, dyes, cellulose nitrate, nitro benzene, nitro chloro benzene, nitro toluene, acrylic fibre, dye intermediates, etc.
We also supply nitric acid with concentration of 55 %, 60 %, 98 % as per your requirement.
‘

